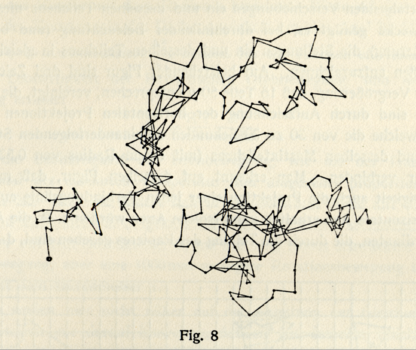
In the 19th century scientists attempt various explanations of the incessant motion of small particles suspended in a liquid.
One possible explanation involves the collisions with atoms. But this explanation presupposes that the hypothesis that heat is nothing else than the motion of atoms is correct.
A confirmation of this hypothesis is expected from the measurement of the velocity of Brownian motion. It should be possible to calculate it from the atomistic theory of heat.
But the attempts to determine the velocity of Brownian motion fail.
 Scene
Scene


 1st Slide
1st Slide
 Branching Point
Branching Point
 Module: Herausforderungen im Labor: Grenzprobleme - Labor
Module: Herausforderungen im Labor: Grenzprobleme - Labor Sequence: 0.0._START_20610_einstieg_grenzprobleme
Sequence: 0.0._START_20610_einstieg_grenzprobleme Branching Point: Borderline Problems of Classical Physics
Branching Point: Borderline Problems of Classical Physics Slide: Ludwig Boltzmann: Lectures on the Principles of Mechanics
Slide: Ludwig Boltzmann: Lectures on the Principles of Mechanics  Back
Back