Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik
In the old quantum theory
...
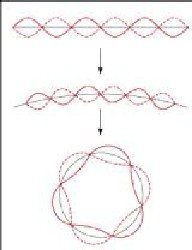
... the atom is viewed like a small planet model where the electrons are circling around the atomic nucleus. Their later (by de Broglie) discovered wave character comes into play in that only such orbits are allowed where an integer number of periods may be placed (in the figure three; shown are two different states of oscillation each).
This is Bohr's atomic model, and the quantum condition (in the later wave picture the integer number of periods per electronic orbit) is named today mostly after Bohr and Sommerfeld.
[ Sitemap ]
[ info ] This website was created by the MPI for the History of Science.
 Scene
Scene


 1st Slide
1st Slide
 Branching Point
Branching Point
 Module: Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik
Module: Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik Sequence: lev0_start
Sequence: lev0_start Branching Point: Particles and Light under Control
Branching Point: Particles and Light under Control Slide: Functional Principle of the Paul Trap
Slide: Functional Principle of the Paul Trap
